Aerosol Cosmetics
Aerosols are scientifically defined as a colloid of fine, solid particles or liquid droplets in air or another gas; a definition that includes human-made products but also natural phenomena like fog. In everyday usage, the term is more commonly used to describe products dispensed from self-pressurised containers such as fly-sprays. This means that pump-sprays or atomisers are not called aerosols even if they produce an aerosol spray in the scientific meaning of the term.
The origins of self-pressurised sprays have been traced back to the nineteenth century but most writers credit the modern development of aerosols to Erik A. Rotheim [1898-1938], a Norwegian chemical engineer and inventor who created a pressurised device to spray wax onto his skis. Rotheim believed his invention had wide applications and in one of his patents suggested this could include cosmetics:
Cosmetic products such as for example liquid or solid brilliantines, pomatums, vaselines, creams, toilette liquids and the like are in accordance with the present method handled in a more practical and hygienic manner than at present.
(US Patent: 1892750, 1933, p. 2)
There was some commercial development of pressurised spray containers in the 1930s – most notably in the production of aerosol fire extinguishers – but it would not be until the Second World War that they were manufactured in large numbers.
High-pressure aerosols
During World War II, Lyle D. Goodhue [1903-1981] and William N. Sullivan [1908-1979], working at the United States Department of Agriculture, developed what became known as the ‘bug bomb’. This high-pressure aerosol delivered a fine spray of insecticide that killed flying and crawling insects. About 50 million of these ‘bug bombs’ were produced for military use during the war (Mina, 1962, p. 1) made under licence by companies such as Westinghouse.
American soldiers, scattered as they are in every climate of the world, face an unseen foe every bit as deadly as the soldiers of the enemy. Men sleeping in tents in the hot countries must be protected against disease—carrying insects. Planes flying from one tropical region to another must likewise be rid of insects. Surprisingly enough, Westinghouse refrigerator engineers provided the answer by developing an “insecticide bomb” from a device they had used in peacetime to charge the compressors of household refrigerators. The “bomb” is a dispenser about the size of the average food can, made to withstand high internal pressure. Upon the turning of a screw, the insecticide emerges as an extremely minute mist of such penetrating power that it reaches into every hole and crevice. Enough insecticide can be released in ten seconds to kill very mosquito in an airplane, and the fumigation can take place even in flight, without in any way disturbing the passengers.
(Westinghouse, 1942, p. 8)
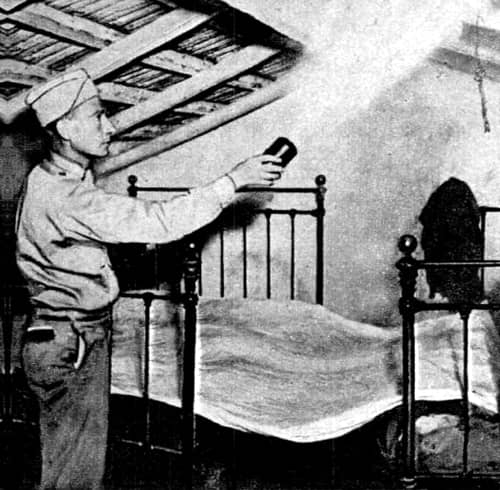
Above: 1946 An American soldier using a ‘bug bomb’ in Algiers to control malaria mosquitoes (Popular Mechanics, 1946).
After the war, aerosol insecticides were made for civilian use. These sold well despite their high cost. However, the weight and cost of the high-pressure containers restricted their application in other areas; a limitation largely overcome by the development of low-pressure aerosols.
Low-pressure aerosols
First introduced in 1947, these aerosols used much lower pressures than previous devices, allowing the use of lighter weight, less costly disposable containers. Commercial aerosols were not introduced into Europe or elsewhere until 1951 so all the early low-pressure devices were American. Early cosmetic aerosols included Gay Manhattan (1946), an aerosol perfume produced by Daggett & Ramsdell of New York and Sonni Foam Shampoo (1948) developed by the Gebauer Chemical Company of Cleveland. Neither product appears to have had much success in the market place; Gay Manhattan was discontinued in 1948 and Sonni Foam Shampoo was abandoned after less than 300 bottles were sold.
Aerosol principles
The basic working principles of an aerosol are relatively simple. When a liquefied gas (propellant) is sealed in a container with a suitable product some of the propellant evapourates and exerts a pressure. When the valve is released this pressure forces the product out of the valve as a spray, foam or stream depending on how the contents were formulated. As the product in the container is used up more propellant evapourates. This fills the growing space and maintains the pressure in the container ensuring an even flow of the product as it is used up.
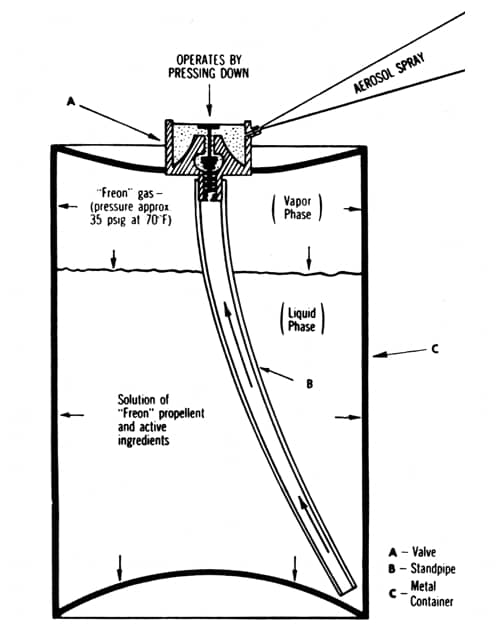
Above: Cross section of a typical two-phase aerosol spray (Sherperd, 1957). In this type of aerosol the propellant and product are mixed together. Some of the propellant evapourates to generate the pressure needed to create the spray.
Early cosmetic aerosols
Despite the numerous defects of early aerosol spray cans – faulty valves, chilling effects and over spraying – an increasing number of cosmetic aerosols were introduced during the 1950s and 1960s, particularly after 1953 when many of the early container and nozzle problems had been dealt with.
As well as their novelty factor, aerosols had a number of advantages when compared to other containers. Products dispensed in an aerosol did not evapourate, were not contaminated with bacteria or dust through use and could not be spilled. This helped offset their higher costs and early teething problems. Aerosols were also ideally suited for television advertising which became increasingly important after 1950; unlike many other products they had ‘live action built-in’. They did have other issues apart from cost – such as dangers from flammable materials and container rupturing – but these were ameliorated through later government regulation.
Despite their enthusiastic promotion by the aerosol industry many cosmetics were not suited to being sold in this form. Make-up was a noticeable omission, with the possible exception of a spray-on leg make-up and although a few skin-care aerosols were developed none produced major sales. There was an attempt at selling a spray on nail polish combined with a guard to cover the rest of the finger which was abandoned. However, some companies had limited successes with an aerosol nail drier which sprayed a thin layer of oil onto the nail polish.
The most successful aerosols cosmetics in the 1950s were hair-sprays and shaving foams with deodorants and antiperspirants becoming more important in the 1960s. Other successful aerosols cosmetics were fragrances and sunscreens.
Shaving foams
In April, 1950, Carter Products, Inc. released Rise, an aerosol shaving foam, the world’s first commercially successful aerosol cosmetic, using a patent they acquired from Reich, Spitzer and Fine (US2655480).
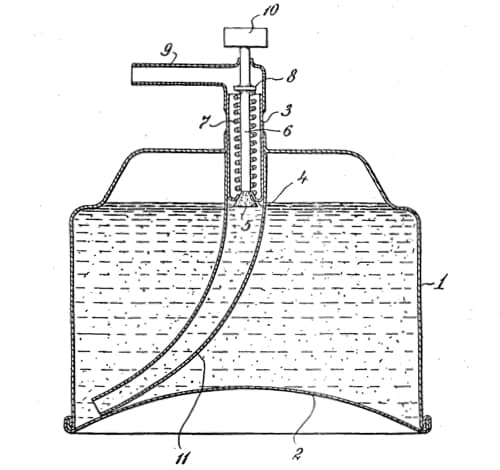
Above: 1953 Drawing from U.S. Patent 2,655,480. Once the patent was granted Carter Products began proceedings for patent infringement against a number of other aerosol shaving foam producers.
Other companies developed similar products and by 1954 it is estimated that between 50 and 60 million of these aerosols were being sold annually in the American market (Zumbro, 1954, p. 262).
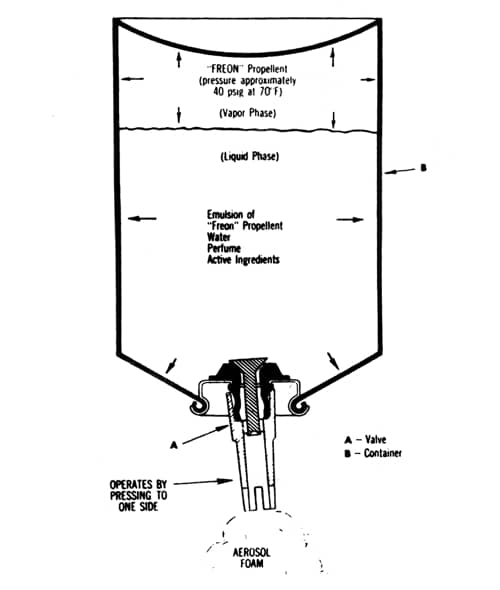
Above: Cross section of a typical foam aerosol (Sherperd, 1957). Made with a lather producing base mixed with a propellant to pressurise the container and generate the foam.
Hair aerosols
A number of different aerosols were developed for the hair-care market including hair sprays and hair tints.
Hair sprays: Also known as hair lacquers, hair sets and liquid hair sets. The first hair sprays were made with shellac dissolved in alcohol. This was unaffected by rain or humidity but was difficult to wash out and the lacquer coating also built up on the hair over time becoming brittle and flaky. Shellac was soon replaced by, or combined with polyvinylpyrrolidone (PVP) which produced a glossy, softer set that could be largely removed by washing or reshaped using a wet comb. Other resins were also used and lanolin, lanolin derivatives, mineral oils, and isopropyl esters of fatty acids were added as conditioners.

Above: 1956 Helene Curtis Spray Net in a purse-sized refillable container, the world’s first refillable aerosol cosmetic.
Hair tints: These could be sprayed onto or brushed through the hair to provide touch-up or instant temporary colour that was removed with the next wash. Conditioning agents could be included with the formulation.
Perfumes and colognes
Until 1952, all aerosols were packaged in metal containers as even low-pressure aerosols were considered too risky to be safely contained in glass or plastic. This changed when Larvex, a water-based mothproofing aerosol, was marketed in glass, followed soon after by a Myna, a glass-cleaner (Mina, 1962, p. 3). Although both of these products used pressures that were less than half that used in low-pressure aerosols – i.e., they were ultra-low pressure aerosols – their manufacturers appear to have been unsure about their safety as they enclosed the glass containers in metal cans.
Fragrance manufacturers followed this lead and by 1953 a number of alcohol-based colognes in glass containers were being sold. These were marketed without protective metal shields. Manufacturers that wished to use higher pressures generally coated the glass in plastic as an additional safety measure.
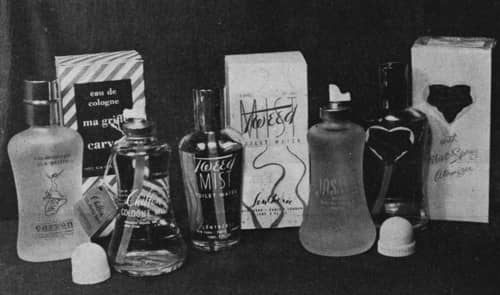
Above: A range of early aerosol fragrances sold in glass containers.
Despite its risk of shattering, glass had a number of advantages when compared to metal containers. It could be made in a wide variety of attractive shapes – an important consideration for fragrances – was free of most of the corrosion problems associated with metals and was unlikely to react with any of the ingredients in the formulation, either the product or the propellant.
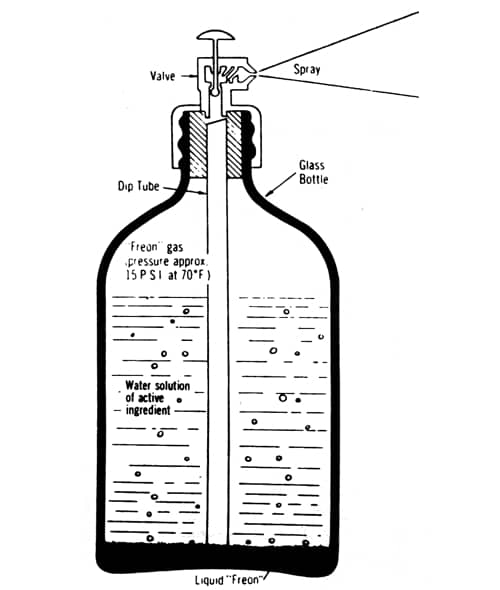
Above: Cross section of a three-phase glass aerosol (Sherperd, 1957). The product dissolved in water or alcohol sits above the heavier liquid propellant. As the product is used up some of the liquid propellant in the bottom of the container evapourates to maintain the pressure needed to create the spray.
Sunscreens
These were sold as sprays or foams. The sprays required some sort of carrier, either alcohol or oil, with the latter having the advantage of being water repellent. Aerosol sunscreens were more popular in Europe, particularly in Italy, than the United States until a cocoa butter aerosol was developed which clung to the skin and was less likely to be blown away by the wind (Root, 1972, p. 478).
Deodorants and antiperspirants
Deodorants and antiperspirants were relatively late entrants into this class of cosmetics. In 1960, Gillette released Right Guard Deodorant with hexachlorophene in an alcohol base as the deodorising agent. It sold well even though it did not control perspiration. Used by both men and women, it was easy to apply and dried quickly. Also, because it did not touch the skin the same container could be used by the entire family.
Antiperspirants were more of a problem. Although physical barriers – like spraying a film of ethyl cellulose on the skin – were tried, a true antiperspirant required the use of an aluminium salt such as aluminium chlorohydroxide. Unfortunately, the formulations that were current at the time were corrosive to metal and clogged the nozzles. The corrosive effect could be controlled by using glass containers but fixing the nozzle clogging problem proved to be more difficult.
In 1967, Carter-Wallace released Arrid Extra Dry, the first true aerosol antiperspirant. It solved the corrosion and clogging problems by using micronised aluminium chlorohydrate dispersed in an oil. As no water was used this reduced the build-up of crystals that had previously blocked the valves (Laden, 1988, p. 7). Arrid Extra Dry was an immediate success and was quickly followed by other manufacturers releasing similar products. Within a few years, aerosols outsold all other forms of deodorants and antiperspirants – roll-ons, pads, creams, squeeze bottles and sticks – in the American market and have remained popular to this day.
Manufacturing aerosols
The production of aerosol cosmetics required specialist equipment so most cosmetic companies elected to have their products manufactured under contract by a specialist. Early manufacturing used either cold-filling or pressure-filling.
Cold-filling: As this method had a fast production rate it was the preferred manufacturing method used for aerosols that were not affected by extremely low temperatures. Unfortunately, this meant that it was unsuitable for water-based aerosols. In cold-filling the product and propellant are both refrigerated in separate tanks and added separately to the container which is then capped and sealed with the valve. The container is then heated in a hot water bath to detect leakages, then tested, packaged and shipped.
Pressure-filling: Although slower this method was used for water-based aerosols and for those affected by lower temperatures such as high-grade perfumes and colognes. In pressure-filling the product is first added top the container which was then sealed with a valve before propellant is forced in under pressure. The filled container was then passed through a water bath to detect leakages, tested, packaged and shipped.

Above: Pressure-filled aerosol assembly line (Mina, 1962). The woman at bottom right is adding empty glass containers into metal cups which then move to the filling station at the lower left. The seated woman to the left is watching the filled aerosols pass through a water bath where they are scrutinised for leaks. At the back of the assembly line are four women testing and packaging the completed aerosols under the watchful eyes of a supervisor. All the women appear to be wearing protective glasses.

Above: Pressure-filled aerosol assembly line (Mina, 1962). This is photograph shows more detail of the pressure filling machines and the water bath used to test for leaks. Protective plastic screens give the operator additional protection from the occasional mishap.
As with other manufacturing process new techniques such as undercap-filling were developed over time.
Temporary setbacks
The aerosol market in the 1950s and 1960s was very buoyant and this also applied to aerosol cosmetics. Many writers of the time were very optimistic about potential developments.

Above: 1955 A range of aerosols produced by Nestle-LeMur using plastic-coated bottles (top row) and metal cans (bottom two rows).
However, in the 1970s, the cosmetic aerosol market was hit with a number of setbacks. These included questions about the safety of aluminium-zirconium salts in aerosol antiperspirants; the long-term effects of inhalation of aerosol products; and evidence that the most commonly used propellants, chlorofluorocarbons (CFCs), were depleting the ozone layer. Added to this were changes in fashion – the most important being a decline in the use of hair sprays as fashion moved towards longer, looser hair styles – and improvements in competing containers such as pump packs and squeeze bottles. However, the cosmetic aerosol industry continues to thrive today despite these and other challenges and has continued to innovate.
Airbrush make-up
An airbrush is a small device that uses compressed air generated from a compressor to spray paint, ink or other materials onto surfaces. Used for many years to apply paint, ink varnish and other liquids it was first used to apply liquid make-up in the film ‘Ben-Hur’ (Metro-Goldwyn-Mayer, 1959) to give the cast and extras a tanned skin.
This idea was subsequently used by the funeral industry, in special FX make-up, in body art, for fantasy make-up and, in more recent times, to high-definition television and digital photography make-up. However, given the need for a heavy air compressor and an expensive airbrush gun their use has been limited to professional make-up artists.

Above: The replicant Pris using an airbrush to spray paint her eyes in the film Blade Runner (Warner Brothers, 1982).
Although airbrushes powdered by an external mechanical compressor produce an aerosol in the scientific meaning of the term they work on a different principle to aerosol spray cans or bottles. However, some cosmetic companies have introduced aerosol spray-on foundations – that mimic the effects of airbrush make-up – in a convenient aerosol spray: just one example of continued innovation in this area.
Updated: 25th June 2017
Sources
Aerosol pioneers. (1956). The American Perfumer & Essential Oil Review. April, p. 6.
Flanner, L. T. (1975). Aerosols. In M. G. deNavarre (Ed.), The chemistry and manufacture of cosmetics (2nd ed., Vol. III, pp. 173-204). Orlando, FL: Continental Press.
Laden, K., & Felger, C. B. (1988). Antiperspirants and deodorants. New York: Marcel Dekker, Inc.
Mina, F. A. (1962). Aerosols. In M. G. deNavarre (Ed.), The chemistry and manufacture of cosmetics (2nd ed., Vol. I, pp. 1-52). Princeton, NJ: D. Van Nostrand Company, Inc.
Root, M. J. (1972). Aerosol cosmetics. In M. S. Balsam & E. Sagarin (Eds.). Cosmetics Science and Technology (2nd ed., Vol. 2, pp. 417-485). New York: Wiley-Interscience.
Sherperd, H. R. (1957). Aerosol cosmetics. In E. Sagarin (Ed.). Cosmetics Science and Technology (pp. 787-839). New York: Interscience Publishers, Inc.
Westinghouse Electric & Manufacturing Company. Annual Report 1942.
Zumbro, F. R. (1954). Aerosol cosmetics. The American Perfumer & Essential Oil Review. April, 261-264.

1943 The ‘bug bomb’ developed by the United States Department of Agriculture.

1947 Daggett & Ramsdell Gay Manhattan Perfume Bomb. Introduced in 1946, it was discontinued in 1948. Daggett & Ramsdell were early investors in aerosols through the patents of George W. Fiero [1906-1969] granted in Argentina (1947) and Panama (1948). The product’s shape meant that it was often referred to as a ‘perfume bomb’.

1949 Gulfspray Aerosol Bomb.

1950 Carter Products Rise. This push-button shaving foam was the first commercially successful aerosol cosmetic. The product was widely copied but was covered by a pending patent (US2655480, 1953) and Carter took a number of other companies to court for infringement once it was granted.

1953 Copper Tan as a cream, lotion and aerosol.

1953 Helene Curtis Spray Net. Spray Net was first sold in a plastic squeeze container in 1951 but the company switch to an aerosol in 1952.

1954 Dorothy Gray Tanning Oil. This advertisement is promoting Crown Cork & Seal Company’s Spra-Tainer. Crown was a major American manufacturer of aerosol spray cans.

1954 Skol Sun-Fluff, an aerosol tanning foam.
1954 Lenthéric Tweed Mist.

1956 Mennen Foam Shave.

1956 Helene Curtis Spray Net Purse/Spray.

1956 Campana Italian Balm Lotion Spray.

1956 Colgate-Palmolive Lustre-Net.

1959 GLO-RNZ Glo-Puff Instant Color in 12 shades. First released in 1958, this may have been the first temporary aerosol hair colourant on the market.

1960 Colgate Dental Cream in tube and aerosol containers.

1965 Gillette Right Guard Deodorant and Sun Up Shaving Foam in what appear to be glass and/or nylon aerosol containers. Although advertise here to men, Right Guard also appealed to women and Gillette also made a version packaged specifically for them.

1966 Carter-Wallace Arrid Spray Deodorant, Cream and Roll-on.

1969 Carter-Wallace Arrid Extra Dry Anti-perspirant, the first commercially successful aerosol antiperspirant.
